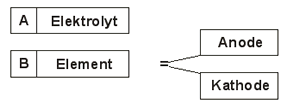
Effectiveness of cathodic corrosion protection
A. Electrolyte
water, soil and atmospheric moisture
B. Element >>> element voltage
A voltage can result in different influences, such as
To suppress this element voltage is the anode and cathode with an insulating material covered, i. e. coated.
Coating materials: Paints, Bitumen, PE insulation
This element voltage is theoretically prevented by the passive corrosion protection. As always damages (Faults) of the passive protection, active protection (CP) must be used in addition. The protection current flows only at the defects in the steel and prevents corrosion.